Bowen's Reaction Series
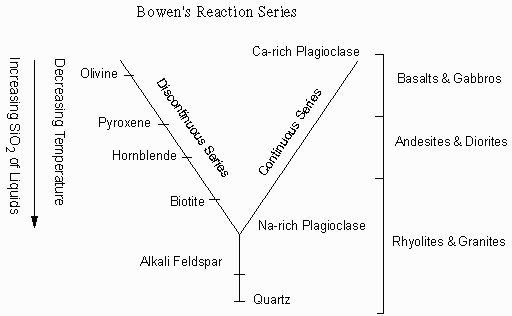
Norman L. Bowen, an experimental petrologist in the early 1900s, realized this from his determinations of simple 2- and 3-component phase diagrams, and proposed that if an initial basaltic magma had crystals removed before they could react with the liquid, that the common suite of rocks from basalt to rhyolite could be produced. This is summarized as Bowen's Reaction Series. |
Bowen suggested that the common minerals that crystallize from magmas could be divided
into a continuous reaction series and a discontinuous reaction series.
|
This generalized idea is consistent with the temperatures observed in magmas and with
the mineral assemblages we find in the various rocks. We would expect that with
increasing SiO2 oxides like MgO, and CaO should decrease with higher degrees of
crystal fractionation because they enter early crystallizing phases, like olivines and
pyroxenes. Oxides like H2O, K2O and Na2O should increase
with increasing crystal fractionation because they do not enter early crystallizing
phases. Furthermore, we would expect incompatible trace element concentrations to
increase with fractionation, and compatible trace element concentrations to
decrease. This is generally what is observed in igneous rock suites. Because
of this, and the fact that crystal fractionation is easy to envision and somewhat easy to
test, crystal fraction is often implicitly assumed to be the dominant process of magmatic
differentiation.
___________________________________________________________________________ Source Link: Magmatic Differentiation ___________________________________________________________________________ More Links to See: What is Bowen's Reaction Series? Bowen's Reaction Series and the Igneous Rock Forming Minerals |
No comments:
Post a Comment